
In most species electrical activity of the nervous system displays transient or sustained rhythmic character. This holds true for electrical signals recorded by various means, from intracranial local filed potentials (LFP) to large surface EEG scalp electrodes, from almost any area of the mammalian nervous system, from the spinal cord to the prefrontal cortex. In addition to this ubiquity of neuronal oscillations, their characteristics (such as frequency, amplitude, regularity or duration) show strong associations with physiological or pathological brain states, behaviour and cognitive operations. These findings suggest that neuronal oscillations emerge from an underlying process or processes fundamental to the ways how brains work. Indeed, the most important elementary network operation of the nervous system, the integration of incoming synaptic activity and its translation to action potentials by a single neuron is decisively dependent on the synchronization within and between incoming activity and fluctuations in the excitability of the neuron. The exact same processes heavily influence the emergence of electrical oscillations recorded in the extracellular space. Hence, studying neuronal oscillations offers a window into the inner workings of brain networks.
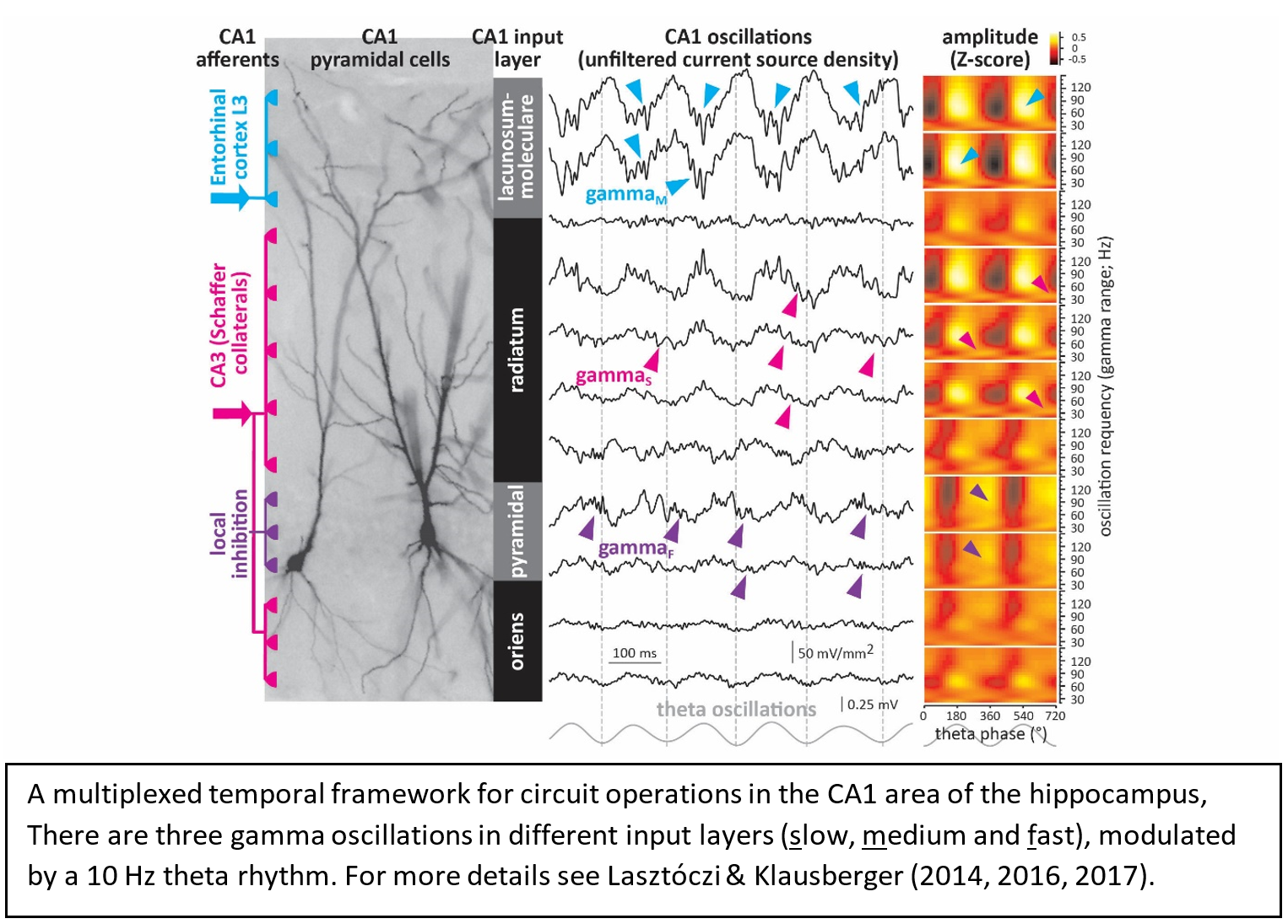
The goal of our research is to understand how network operations and ultimately the regulation of behaviour emerges from the collective temporal dynamics in neuronal circuits of the rodent brain. We take an experimental approach based on recording extracellular field potentials from awake animals by multi-electrode arrays which allow analytical separation of neuronal oscillations emanating from various anatomically segregated and physiologically relevant sources. Our primary concern is neuronal activity in the CA1 area of the hippocampus, which lies at the confluence of input streams carrying information on current experiences and memories and plays key role in spatial navigation and episodic memory. The coordination of these anatomically segregated input streams and the local network operations of the CA1 area is supported by a complex system of interacting neuronal oscillations that provide a multiplexed temporal framework for information processing.
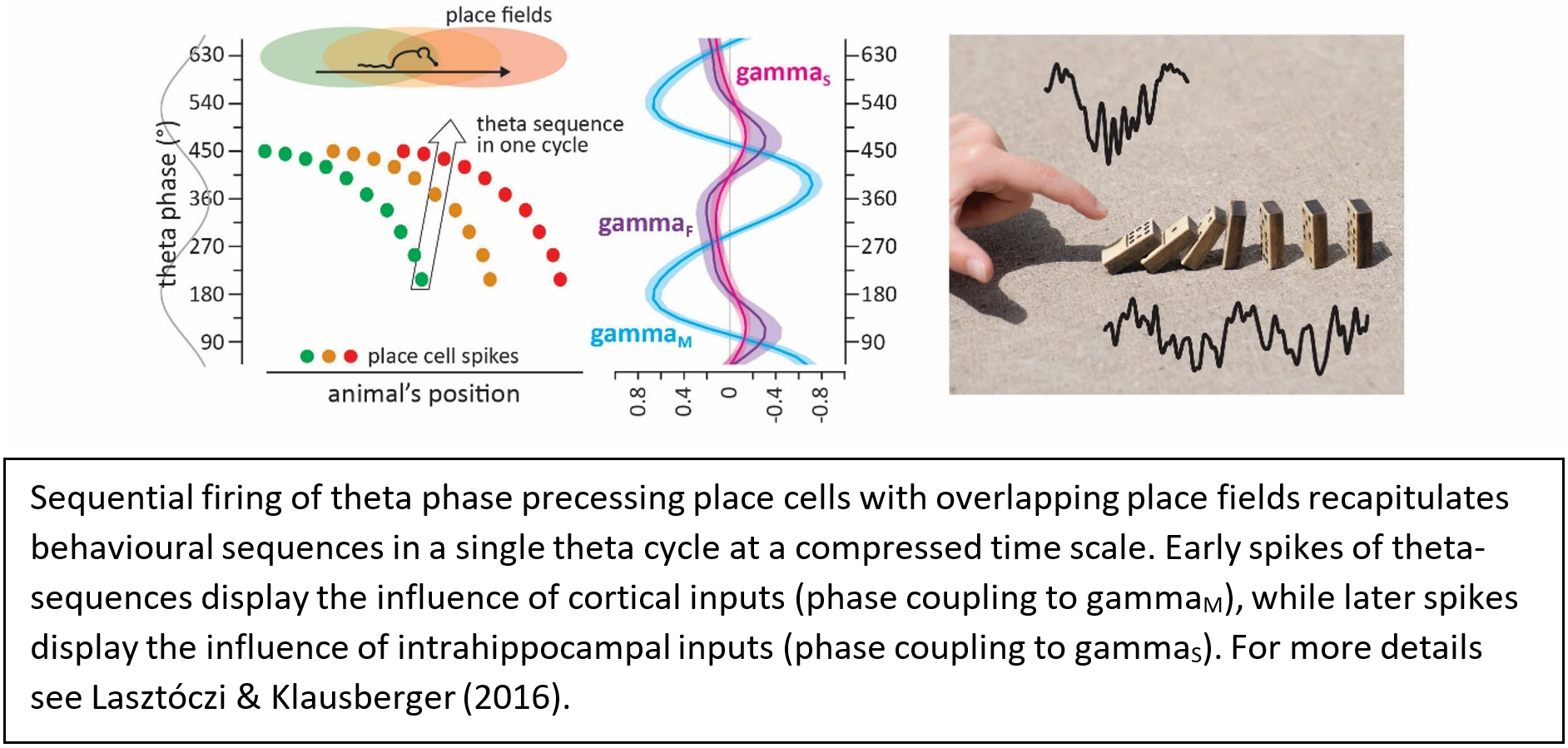
Building on our understanding of this complex temporal framework we put forward to decipher how neuronal oscillations coordinate the spike timing of neurons. In the mammalian cerebral cortex long range communication between brain areas and network operations within local circuits are mainly mediated by large populations of sparse firing glutamatergic projection cells (pyramidal cells). Again, using the CA1 hippocampal circuitry as a model system we record the spiking activity of collectives of pyramidal cells to understand how their spike timing (referenced to neuronal oscillations) interacts with behavioural correlates (spatial firing fields).
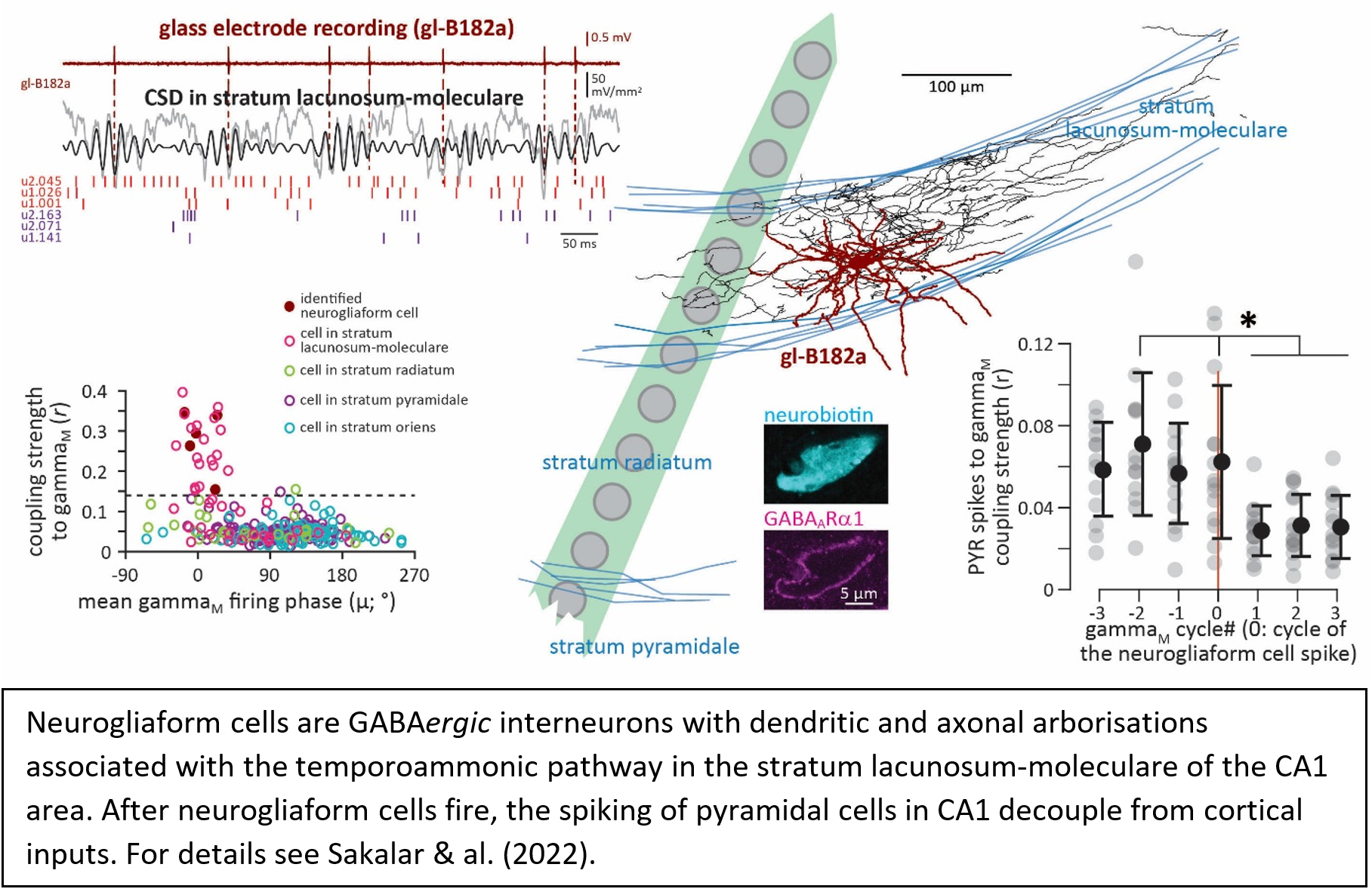
In addition to the excitatory cells, the cerebral cortex contains a smaller, yet highly heterogeneous population of GABAergic inhibitory cells, most of which are interneurons with local axonal arborisations that typically follow the cortical stratification. GABAergic cells often fire in a rhythmic fashion and are classified into several types, featuring distinct connectivity and molecular and biophysical properties, and therefore their study warrants careful identification of the recorded cell. We use the juxtacellular recording technique (often combined with multi electrode array recordings) in awake animals to study how identified GABAergic cell types contribute to the generation of neuronal oscillations, how they coordinate the activity of projections cells, and how they regulate network operations.





